Optimising clinical trials: enhancing engagement and outcomes through tailored technology
Central Nervous System (CNS) drugs in Phase 2 and 3 clinical trials have a high failure rate, reaching up to 85%, second only to oncology [1]. The cost per failure in this space is estimated at $1 billion, a substantial cost borne by pharmaceutical companies. Most importantly, such failures impact people living with CNS conditions, who await breakthroughs and new treatment options.
Given the urgent demand for drugs to address multifactorial neurodegenerative challenges and the potential impact of orphan drugs targeting rare conditions, it is crucial that we assess the challenges associated with trial execution that often result in unfavourable outcomes:
a – Trial failure can be linked to an inadequate study design, where endpoints rely on subjective reports from participants and observations made by clinical investigators [1].
b – David Reese, Amgen’s Executive Vice President of R&D, emphasises the limited understanding of neurological disorders and the complex development requirements for drugs in this field [2].
c – Additionally, participant engagement is a significant issue, with over 80% encountering challenges in retention. One in every five clinical trials is prematurely terminated due to a decline in participant numbers [3].
Trial failure not only denies patients access to much-needed treatments, but also adds to the overall cost of the failed trial, previous trials, and the missed opportunity of pursuing viable alternatives.
For the last 8 years, Beats Medical have addressed these challenges, defining the following hypotheses:
– Will the decentralisation of various aspects of a study or clinical trial considerably alleviate the burden on patients and, consequently, enhance participant retention and reduce overall trial costs?
– Will a digital platform, tailored to the person with the condition, deeply enhance our understanding of constantly evolving symptoms?
Optimising Clinical Trial Outcomes
The COVID-19 pandemic has expedited the adoption of technology and improved digital literacy among patients. While it is assumed that remote trials alleviate the logistical burden on participants, some have reported difficulties, suggesting the presence of “digital overload” [3]. Embracing technologies familiar to participants, such as BYOD (bring your own device), can help alleviate some of these challenges by reducing the feeling of being inexperienced and the fear of failure [4] [5]. The recent first eCOA Forum examined the requirements and benefits for sponsors to explore through a BYOD setup [6].
Simultaneously, it is becoming increasingly evident that remote trials can provide reliable and intentional data collection from real-world settings. By embracing digital platforms, companies can tap into the potential of gathering highly detailed data that surpasses the limitations of in-clinic methods.
At Beats Medical, we like to describe this shift as a move “to measure in millimetres, not meters” leveraging commercial smartphones, their sensors capabilities and Beats Medical’s trained Artificial Intelligence (AI) models.
Beats Medical has developed a tailored platform for pharmaceutical companies to:
1 – Avoid the upfront costs of building a platform from scratch, easily accommodate growth and adapt to changing market needs, based on their digital health strategy.
2 – Define objective endpoints and build a deeper understanding of the conditions being researched, while defining their strategy for future commercialisation of breakthrough therapeutic programs.
3 – Differentiate from other competitors in the market by providing a unique commercialised product under their own brand that delivers added value to their customers; utilise it to conduct studies and devise their strategy around it that can combine drugs with digital intervention.
Our platform can be deployed to target the most common symptoms of any central nervous system condition or rare disease, such as walking, hand movements and speech.
We ensure consistent and dependable results through the standardisation of supported digital assessments, namely electronic Patient-Reported Outcome Measures (ePROMs). They are delivered to the user’s mobile device, whether it be Google’s Android or Apple’s iOS, with no additional hardware required. The most common symptoms can be evaluated with a range of assessments, digitised with a market-leading level of precision, including:
- Walking: Timed Up & Go, 2-minute walk test, 6-minute walk test, timed 25-foot walk
- Hand movement: 9 Hole Peg Test, Spiral Hand Drawing and others
- Speech: Sustained Vowel Phonation, Breath Control and others
- Surveys and Wellbeing
Given the diverse range of smartphone capabilities, Beats Medical have developed algorithms, enhanced by AI, to ensure consistent and precise data collection across all supported iOS and Android devices. Throughout our research process, we discovered that individuals with impaired walking may not find the iOS and Android platforms reliable. We worked relentlessly to overcome this limitation, to ensure our technology delivers dependable outcomes for these populations. We established a comprehensive validation process that incorporates a series of tests for each measurement conducted. This involves real-world observations using video recordings, usability testing and recording visual elements displayed on the screen to ensure consistency across all devices.
Remarkably, our technology, which is built on top of the existing Apple and Google offerings, has exceeded their standard measurements by achieving an impressive 97.5% accuracy in detecting steps for individuals with impaired walking. By harnessing the capabilities of Beats Medical’s platform, companies conducting studies can acquire a comprehensive understanding of the multifaceted aspects of a disease. Our platform empowers researchers to delve deeper into the complexities of various conditions, enabling them to make well-informed decisions backed by precise and reliable data.
Enhancing Patient Experiences
To address retention and engagement challenges, we have placed our focus on driving behavioural change and enhancing the user experience by capitalising on the devices and patterns that individuals are already familiar with. Participants want to consume health-related technology in the same way they already consume their favourite digital products. This approach has produced remarkable outcomes, notably a 102% increase in physical activity among our CNS users over a 14-month duration.
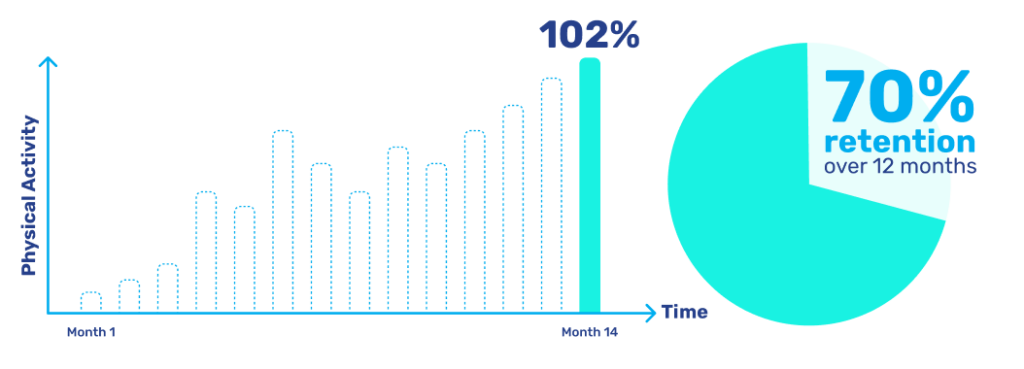
Furthermore, we have achieved an impressive 70% retention rate over a 12-month period, ensuring reliable data collection and sustained commitment from participants throughout each study. These advancements pave the way for faster and more efficient clinical trials, significantly reducing the likelihood of prematurely terminated studies caused by a decline in participant numbers.
Joining Forces for Unlocking New Therapies
The digital revolution has ushered in a new era for clinical trials and therapies, offering transformative solutions to the challenges faced by the pharmaceutical industry, particularly concerning the limited understanding of multifactorial neurological and rare disorders.
The platform’s advanced capabilities require no additional hardware other than a smartphone, providing pharmaceutical companies with the tools to navigate various phases of the process, from real-world data collection to the successful commercialisation of novel therapies.
We firmly believe that technology is the essential driver required to accompany this journey, enabling companies to demonstrate efficacy, obtain regulatory approval, reduce costs, and engage patients effectively. By embracing a unique approach, companies can enhance the likelihood of achieving new and innovative solutions for patients, ensuring greater success in their treatments.
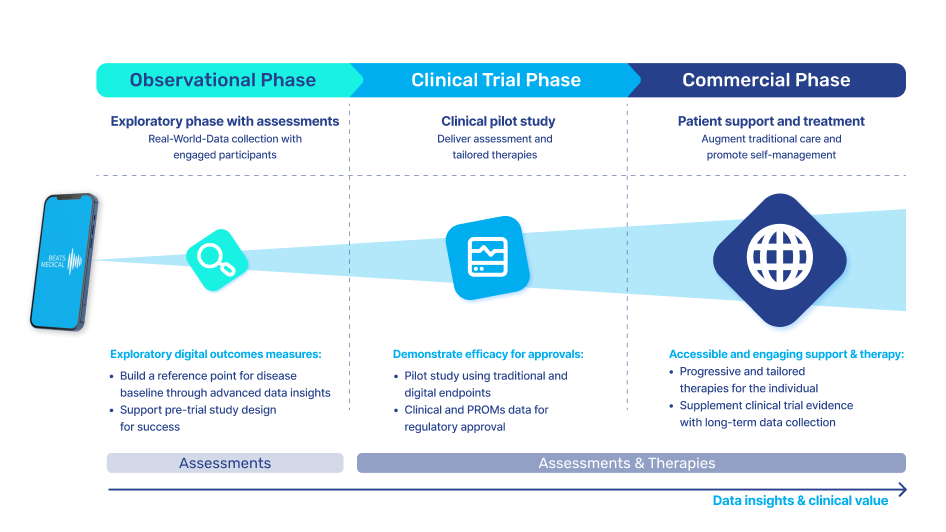
Our long-term vision for our partners also includes the validation of the digital biomarkers of the future. These biomarkers hold tremendous potential to provide unparalleled insights into the emergence and progression of diseases. By harnessing the power of digital assessments and biomarkers, supported by AI, companies can unlock new avenues for revenue generation, expedite time-to-market, and explore opportunities to diversify their product portfolio.
In future articles, we will delve into how digital assessments, such as ePROMs, can serve as intelligent lenses for comprehending complex diseases and accelerating breakthroughs in drug development.
We invite you to join us on this exciting journey as we redefine the future of clinical trials and work together to improve patient outcomes in CNS and rare conditions.
To learn more about our innovative platform and explore partnership opportunities, visit beatsmedical.com or reach out to andrew@beatsmedical.com.
References
[1] WCG – CNS Trial Failure Rates High As Need for New Drugs Grows. https://www.wcgclinical.com/insights/cns-trial-failure-rates-high-as-need-for-new-drugs-grows/
[2] Global Business Reports – United States Biopharmaceuticals 2020 – https://www.gbreports.com/files/pdf/_2020/US_Pharma_2020_-_Web_Version.pdf
[3] Digital Therapeutics and Decentralized Trials: A Match Made in Clinical – https://www.appliedclinicaltrialsonline.com/view/digital-therapeutics-and-decentralized-trials-a-match-made-in-clinical
[4] Donnelly S, Reginatto B, Kearns O, et al. The burden of a remote trial in a nursing home setting: qualitative study. J Med Internet Res. 2018; 20(6):e220.
[5] Coyle, J, Rogers, A, Copland, R, De Paoli, G, MacDonald, TM, Mackenzie, IS, et al. Learning from remote decentralised clinical trial experiences: a qualitative analysis of interviews with trial personnel, patient representatives and other stakeholders. Br J Clin Pharmacol. (2022) 88:1031–42. doi: 10.1111/bcp.15003
[6] The First eCOA Forum Examines BYOD and Item Skipping – https://www.appliedclinicaltrialsonline.com/view/the-first-ecoa-forum-examines-byod-and-item-skipping


